Deep understanding of lithium evolution from graphite negative electrode: detection, quantification, and mechanism
Under normal working conditions, lithium ions will be embedded in the graphite negative electrode intercalation. However, due to kinetic or thermodynamic limitations such as fast charging, low temperature, overcharging, or design defects, metallic lithium will precipitate on the surface of the graphite negative electrode, causing the graphite potential to drop to 0 V vs Li/Li+. Long term lithium deposition on the surface of graphite can lead to capacity decay, and in severe cases, it can puncture the separator and cause internal short circuits, leading to thermal runaway and endangering the safety of the battery. However, there is still no comprehensive solution on how to detect, quantify, and clarify the mechanism of lithium deposition.
Taking graphite/lithium half cells as the research object, a deep understanding of lithium deposition on graphite electrodes in lithium-ion batteries was obtained through lithiation (corresponding to half cell over discharge). The article includes five parts that combine experiments and numerical simulations: 1) Extracting lithium deposition signals from graphite voltage curves; 2) Identification of lithium stripping process based on in-situ heat generation curve; 3) Quantify the reversible efficiency of lithium deposition/stripping during the cycling process; 4) Clarify the lithium precipitation mechanism through disassembly and characterization; 5) Reconstruct lithium precipitation process based on finite element model.
The lithium deposition in lithium-ion batteries poses a serious threat to their safety, so it is crucial to detect and quantify the lithium deposition and understand its generation mechanism. The author added in-situ heat generation identification of lithium deposition/stripping processes based on electrochemical signal detection in previous studies. The lithium stripping process is a reversible deposition process, where the precipitated lithium is re embedded into the negative electrode without causing capacity decay. Therefore, quantifying the reversible efficiency of this reversible process is also beneficial for clarifying the impact of lithium deposition on capacity decay. Finally, the lithium evolution process was reconstructed by combining finite element analysis with experiments, clarifying the mechanism of lithium evolution.
1) Extracting lithium deposition signal from graphite voltage curve
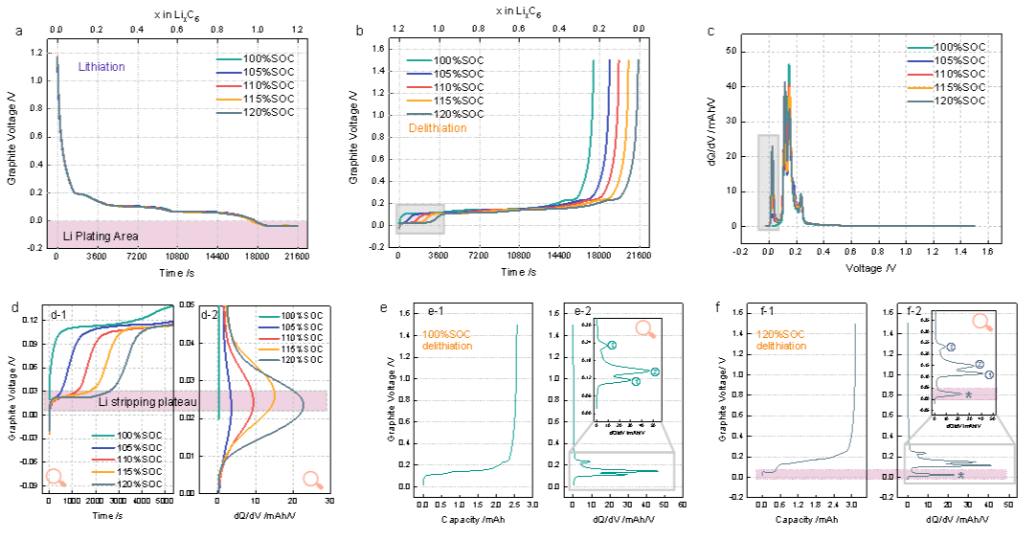
Figure 1: Extraction of lithium deposition signal from graphite electrode voltage curve and IC curve. (a) Graphite lithium insertion voltage, (b) graphite lithium removal voltage, (c) IC curve in Figure b, (d) local magnification of Figures b and c, (e) correspondence between lithium removal voltage and IC curve at 100% SOC, (f) correspondence between lithium removal voltage and IC curve at 120% SOC
2) Identification of lithium stripping process based on in-situ heat generation curve
Based on a button type battery calorimeter, the author obtained the variation of battery heat generation curves for different SOC (see Figure 2). It can be found that there are three heat generation peaks in the battery without lithium deposition, corresponding to the delithiation platform; And the battery that undergoes lithium deposition has an additional heat generation peak "*" during the charging process, corresponding to the stripping platform, and the more lithium is deposited, the more lithium can be reversibly stripped, corresponding to a higher heat generation peak. In addition, the author also found that the peak heat generated by lithium stripping weakens the first lithium insertion peak, which can also serve as a marker for detecting lithium deposition.

Figure 2: In situ heat generation and voltage variation curves during 1C lithium removal process, as well as the proportion of peak heat generation
3) Quantify the reversible efficiency of lithium deposition/stripping during the cycling process
The author first calculated the content of reversible lithium (stripped lithium) through differential voltage method, and then obtained the total coulombic efficiency and the coulombic efficiency of lithium deposition/stripping. It was found that the decrease in reversible efficiency of lithium deposition/stripping after 20 cycles of lithium precipitation was the reason for the decrease in total coulombic efficiency, indicating that irreversible capacity loss has caused a decline in cycling performance

Figure 3 Determination of reversible efficiency of lithium deposition/stripping.
4) Clarify the mechanism of lithium precipitation through disassembly and characterization
Based on the above experimental research, the author has obtained the mechanism and process diagram of lithium evolution, as shown in Figure 4
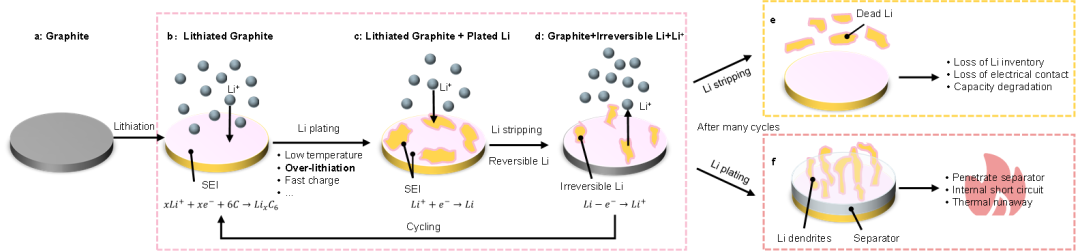
Figure 4 Schematic diagram of lithium precipitation process
5) Reconstruction of lithium precipitation process based on finite element model
Finally, a 2D finite element model was established for the graphite/lithium half cell, and it was found that the lowest graphite potential, highest lithium deposition current, and thickest lithium deposition thickness all occurred at the graphite/separator interface.

Figure 5 shows the finite element model results of lithium evolution at 0.2C and 120% SOC: from top to bottom, the lithium evolution current, graphite voltage, and lithium layer thickness are shown.
conclusion
This article detects lithium precipitation on the surface of graphite electrodes from the perspectives of electrochemistry and heat generation, without causing reversible damage to the battery. The study found that there is a distinct heat generation peak in the lithium stripping process that is different from the de lithiation process, and this peak weakens the normal de lithiation heat generation peak, providing a new detection method for lithium deposition in graphite negative electrodes.





